Chapter 6
The Costs of Perovskites: Sources and Reductions
Technical capabilities, power output, and PCE inform PSC device performance. However, additional considerations govern the technologies’ performance in large-scale deployments.
Materials Costs For Perovskite Solar Cell Technology
No one is going to build a solar panel out of diamonds. To create a valid competitor to current commercial panels there have to be noticeable savings in as many points of the supply and logistics chain as possible to offset the economies of scale enjoyed by the incumbent. Especially one as entrenched as the current crystalline silicon panel industry.
For example, taking into account the preference for very high-purity precursors to limit the potential for defects caused by unwanted elements in the crystal, perovskite precursor inks are quite reasonable in cost. Approximately $250 USD in 2022 will provide enough precursor ink to cover 1 square meter of surface area via a non-spin-coating production method. However, compared to the approximately $100 USD/ m^2 for pure polysilicon in 2022 ($43 USD/kg, 1.16 kg/m^2 for a 0.5mm thick panel plus ~50% wastage from necessary cutting off from the source ingot) perovskites are seemingly at a disadvantage. When you account for the economies of scale, however, adjusting for the relative number of produced silicon solar cells compared to halide perovskite solar cells, it becomes reasonable to posit that there are large savings to be had as perovskite production scales up to match.
Economies Of Scale: Rapid Production Of Perovskite Films For Low-Cost Solar
Where perovskites have the best chance at an early competitive advantage over traditional solar technologies is in production speed. Roll-to-roll manufacturing with solution-based inks has the potential to be rapidly scalable and inexpensive, not only due to the faster production speed but also the amount of production that can be completed under standard atmospheric conditions and temperatures.
Without the need for the controlled vacuum environments of gas/vapour deposition or even worse, high-vacuum for plasma doping, operating costs for perovskite fabrication can be kept low while keeping throughput high. For example, even very unoptimized small batch fabrication costs below $40 USD/ m^2.
Perovskite fabrication is not all benefits, however, since faster production requires less time spent on certain steps such as crystallization and annealing of the crystals to minimize surface defects and grain boundaries that could be detrimental to solar cell operation. To counter the high number of surface defects and hopefully prevent any faulty panels from being sold, there needs to be some form of quality control testing happening on the line itself. This isn’t unique to perovskites, though, as quality assurance testing is common on basically every factory line around the world for every product.
There are many possibilities for the kind of testing that can be done but, understandably, most procedures require the device to be operating under expected conditions, which therefore requires non-destructive testing.
For solar PV this means either sunlight or something as close to sunlight as possible (such as illumination from a solar simulator). Even some non-electrical data collection needs photon illumination, such as photoluminescence scanning to find defects within the cells where poor bonding between layers might have occurred during manufacturing.
These testing methodologies are useful for lab scale cell testing, and doubly so for trying to test any panel scale devices, because as you scale up the size of your devices you want to keep any destructive quality assurance testing to an absolute minimum. This means that a large body of data that links failure modes of panels and cells to certain outputs from the non-destructive methods is required in order to reliably produce high-quality panel-scale perovskite PV. But with the vast field of potential chemical make-ups of perovskite devices, it is extremely time-consuming to characterize the behaviour for every formulation which is why as of yet there is no widely accepted standard for panel testing as a method to predict failure modes of commercial perovskite devices.
As certain formulations become more common in achieving desired properties in the lab, these types of perovskite PV will become the ones most likely to allow proper characterization to inform the creation of acceptable and reliable standards for non-destructive testing for quality assurance and control for commercial perovskite PV panels.
Maximizing Lifespan For Stable Perovskite Solar Cells
Beyond materials and production, however, another important factor in whether a new technology is considered a good investment is its lifespan of operation. The longer a solar cell user can run what they’ve bought, the more time is spent with the device completely paid off and generating free electricity.
Here is where things get difficult for perovskites, however. Historically, perovskite solar cells have been extremely short-lived. Whereas regular silicon solar cells would normally be guaranteed for 20 years and expected to operate likely for 30 years, perovskites in the lab up until very recently could be expected to last for hours or even minutes.
What contributes to this shocking lack of long-term stability in perovskites? A pithy response would be that a shorter list might be what does not contribute. But as we have all been told since childhood, nothing worth doing is easy.
UV Degradation Of Halide Perovskite Solar Cells
Exposure to UV frequencies is known to cause degradation potentially within tens of hours to some perovskite formulations. The degradation occurs most severely at the interface between the perovskite and the TiO2-based electron transport layer (ETL) but reduces when an Al2O3 ETL is used instead.
An interesting occurrence is that the damage from UV exposure can be partially regenerated by the heat from standard sunlight. This effect is related to our earlier discussion of how bulk defects are not responsible for significant perovskite performance decreases. Since a perovskite’s components are fairly mobile within the crystal structure, adding energy in the form of heat allows the crystal to relax from a high-energy disrupted state from the defects back into the lower energy state of the perovskite crystal, pushing the efficiency back up but not quite to the original level of the pristine cell.
Oxygen Reactivity: Problems And Uses For Perovskite PV Technologies
Pure oxygen is a constant concern for almost everything created by humans, and perovskite solar cells are no exception. As oxygen reacts with an organic halide perovskite it has a strong preference for stripping hydrogen atoms from the organic A components. This removal of hydrogen leaves several reactive species behind that proceed to react with everything around them and disrupt the semiconducting properties of the local crystal.
As with most chemistry, this reaction occurs faster and more frequently at higher temperatures which understandably is a problem for solar cells, requiring very thorough encapsulation of the device from the local environment to prevent it.
Oxygen is not entirely detrimental, however. When there are no organic components to react with, oxygen can actually be a useful additive to help “fill in” defects in the perovskite crystal, both bulk and surface, through a process called passivation.
This helps not only to increase the performance of the cell by reducing nonradiative recombination in certain types of defects but also to prevent some types of degradation due to a decreased number of active chemical sites within the crystal.
Water Solubility/Reactivity: Considerations For The Formation Of Perovskite Structures
Atmospheric moisture is more than simply a vector for delivering reactive oxygen to a perovskite; it can result in detrimental reactions of its own.
Foremost is the fact that lead halide perovskites are slightly soluble in water. Therefore too much exposure can lead to the perovskite layer dissolving out from between the hole and electron transport layers.
However, it isn’t quite as simple as preventing all water contact. In the proper concentrations, moisture exposure of perovskites during the fabrication process can prevent pinholes in the perovskite films, increase crystal density and reduce locations where non-radiative recombination can occur.
Denser crystals with fewer defects not only have better performance but also show a longer lifespan in testing, so there is a definite potential for risking the usage of water in the production process before the final encapsulation of the finished product.
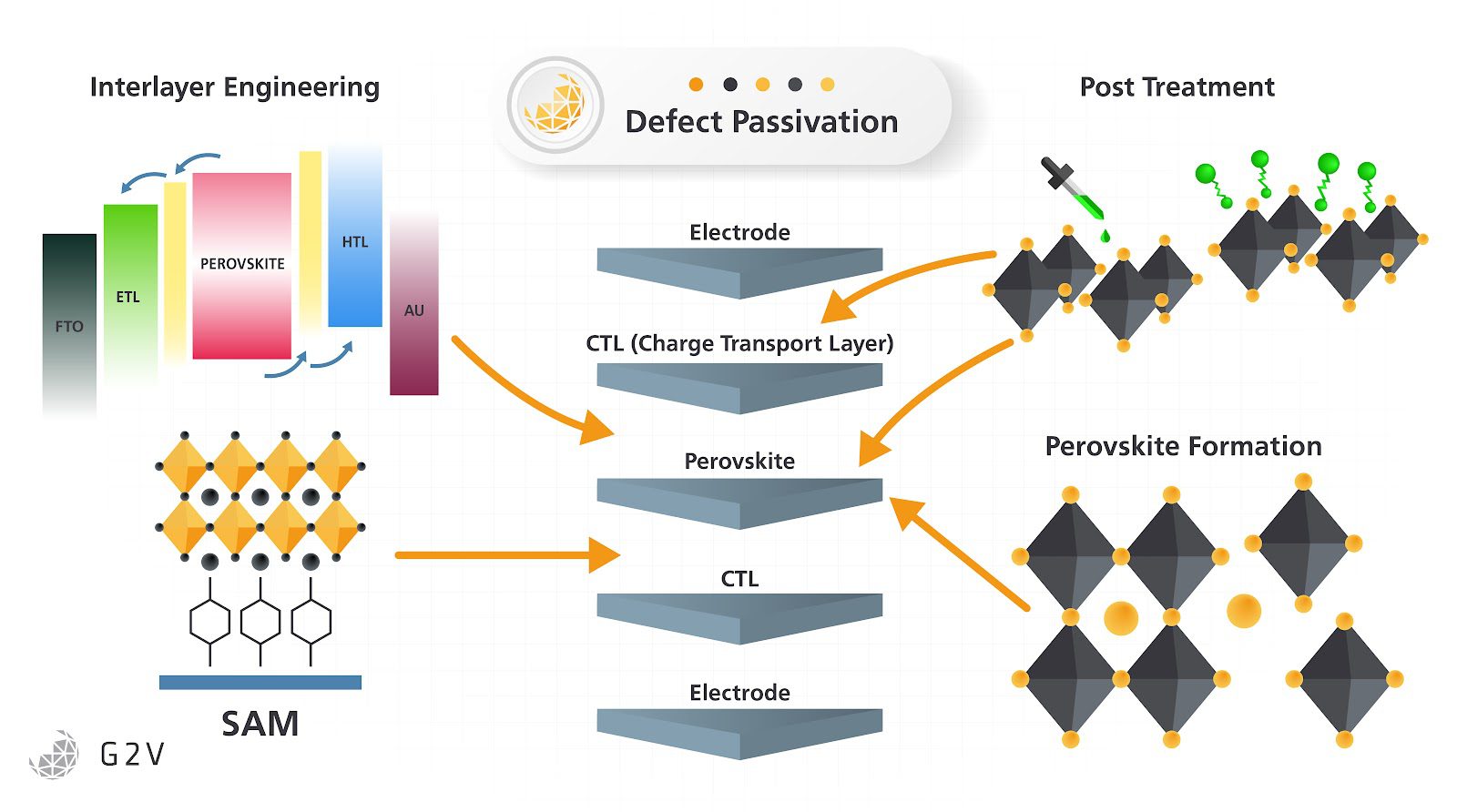
Surface Defect Passivation: A Path Towards Stable Perovskite Solar Cells?
With all these avenues for degrading perovskite devices, how do researchers and producers aim to keep their designs functioning for the 20 to 30-year lifetime the public expects from solar panels?
There was an example we discussed above where oxygen could be used in inorganic perovskites to passivate the crystal. Passivation is the same process that keeps stainless steel or aluminum from rusting, where a reaction between a specific molecule and the device/object occurs that blocks reactive sites on the material, preventing them from acting as sites for reactive chemicals to attack and damage the device/object.
What this molecule is depends on the material you are attempting to passivate but it must be stable enough after reacting with the perovskite surface that it is unable to be dislodged by anything it might encounter in daily use, whether that is temperature, UV radiation, humidity, or chemicals.
Additives For Improved Stability Of The Active Layer In Perovskite Pv Cells
While passivation is the concept of creating a barrier on the surface of a material after it is shaped to prevent future degradation, it is also possible to use less reactive chemicals to exert physical stresses upon the structure of the perovskite during formation to achieve the desired crystallinity. This is to promote the growth of larger crystal grains which lower the number of surface and bulk defects within the device while also minimizing the potential for damage from reactive chemistry.
As an example of the application of unusual chemistry to perovskites, it is possible to promote some perovskites to grow larger crystals during formation using the addition of capsaicin in regulated concentrations. As stated earlier, larger crystal grains equal fewer surface defects in the form of grain boundaries for free charges in the solar cell to be trapped and recombined.
Caffeine is another unexpected additive that has beneficial properties for perovskites. The addition of caffeine to the precursor ink during crystallization acts as an energy barrier for the crystallization, slowing down the rate of crystallization but increasing the quality and density.
By increasing the crystal size and reducing bulk defects within it, ion mobility is reduced. This stabilizes the crystal at higher temperatures and allows a perovskite cell to perform at higher efficiencies under intense sunlight even with the decrease in conductivity due to the suppression of ion transport from normal levels.
It is also possible to simply add a different, large physical 2D crystal to a perovskite to act as a stabilizing feature and a method for improving the conduction of charges between the intrinsic perovskite layer and the electron transport layer.
For example, the usage of graphene for this purpose is very effective, but also currently very expensive and unlikely to improve the commercial validity of the device unless there are significant changes in the price of graphene or other 2D materials. So while there are ways to create improvements or offset shortcomings, it is important to weigh these processes against their future economics.
However, even with all the various ways for perovskite solar cells to fail, there are just as many potential paths to fortifying and stabilizing them against the elements.
Recent publications have showcased extremely impressive results in longevity testing such as the 1000h damp heat examination required by the IEC testing standards as mentioned above. And with further improvements in accelerated aging testing, it is possible to iterate through lifetime testing at a much faster pace than before, accelerating the rate of testing new formulations and constructions until a truly competitive version is found.
As for how competitive perovskites already are compared to other technologies, our next chapter on the energy efficiency of perovskite solar cells, shows why researchers are so convinced that perovskites are a contender to supplant traditional solar cell technologies.