What is the Photoelectric Effect?
The photoelectric effect, where electricity is generated from a material when it absorbs light, was first discovered in 1887 by Heinrich Hertz. He saw that the energy needed to make a spark between two electrodes changed when he shone UV light on them.
Digging deeper, scientists found that electrons (tiny negatively charged atomic particles that are the stuff of electricity) were freed from metals with light shining on them. Since then, we’ve come a long way, with contributions to understanding from intellectual giants like Albert Einstein and Max Planck, who helped us understand light as both a particle (called a photon) and a wave. This in turn helped us get a grasp of why and how light could knock out electrons from materials.
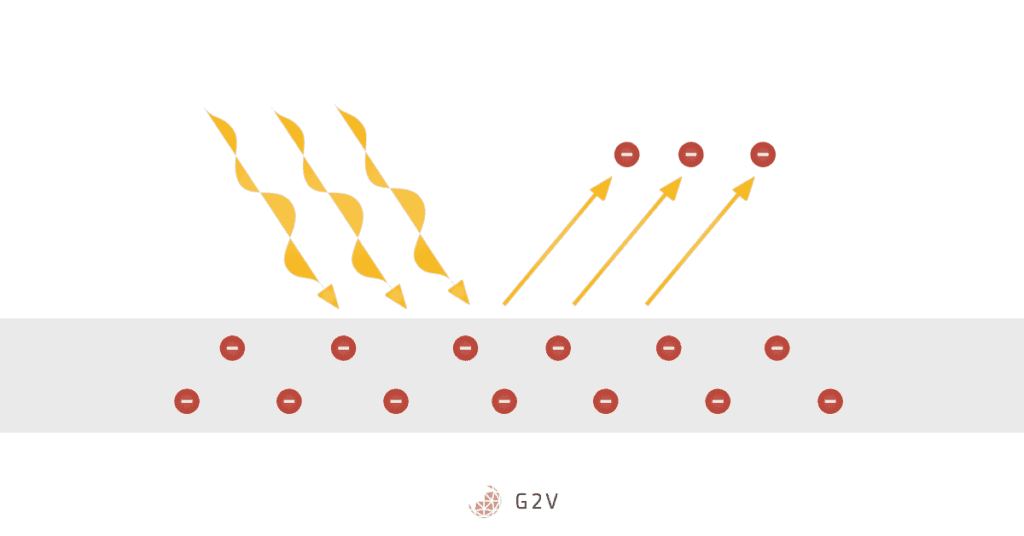
What is The Photovoltaic Effect?
The photovoltaic effect is closely related to the photoelectric effect, with a critical difference. In the photoelectric effect, electrons are emitted into space. But, in the photovoltaic effect, electrons enter what we call the conduction band of the material. Since the photovoltaic effect doesn’t require breaking an electron completely free of a material, it requires less energy, and thus can occur more often.
What do we mean by the “conduction band”? Well, there are two main bands of energies that electrons can have. One range of energies, where an electron is bound around a single atom or molecule, is called the valence band. The other, where the electron is free to move from one atom or molecule to another, is called the conduction band. We’ll discuss this a bit later, but the general idea is that when an electron is in the conduction band, we can conduct electricity, or have electrons move about more freely.
There’s a second requirement for the photovoltaic effect, though. Once an electron is in the conduction band, for the photovoltaic effect to occur, the electron has to move under the influence of an electric potential (or voltage). We’ll see later that this potential can be produced in semiconductors by putting two different, specific materials in close contact.
Electrons that move away from their parent material create a charge difference between where they are, and where they were. This charge difference generates a voltage, much like that across the terminals of a battery, and the moving electrons make up an electrical current. Current and voltage give us electrical power, or energy over time.
The photovoltaic effect is the more practical way we convert solar energy into electrical energy. It’s what solar cells rely on.
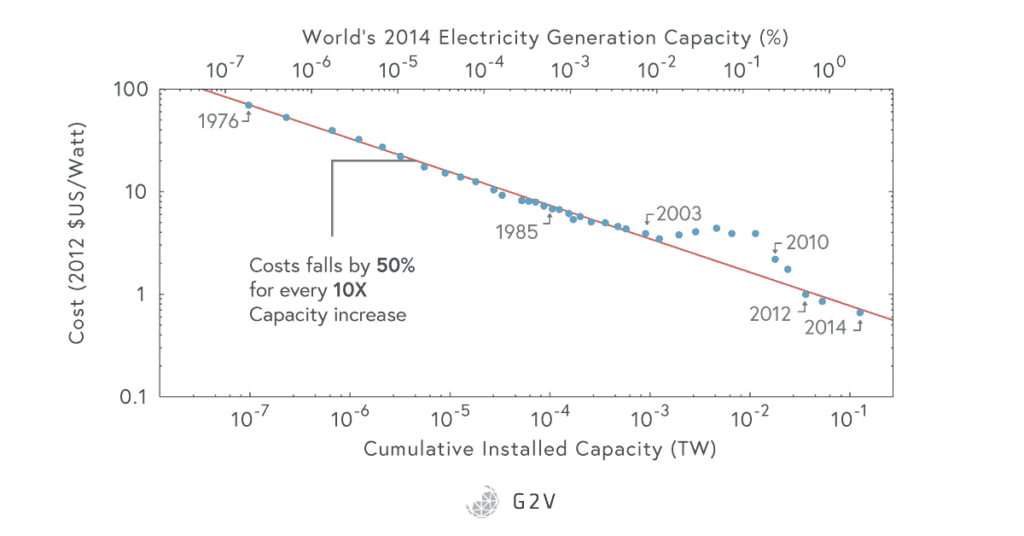
The first photovoltaic cell was made at Bell labs in 1954, and it could only convert about 4% of sunlight into electricity. Today, we’re doing much better, with commercial conversion efficiencies closer to about 20%, and research efficiencies pushing much higher, as shown in the NREL graph below. They’ve also gotten a lot cheaper, too, with cost decreasing by about 10% every year. There are many growing advantages to using solar cells.
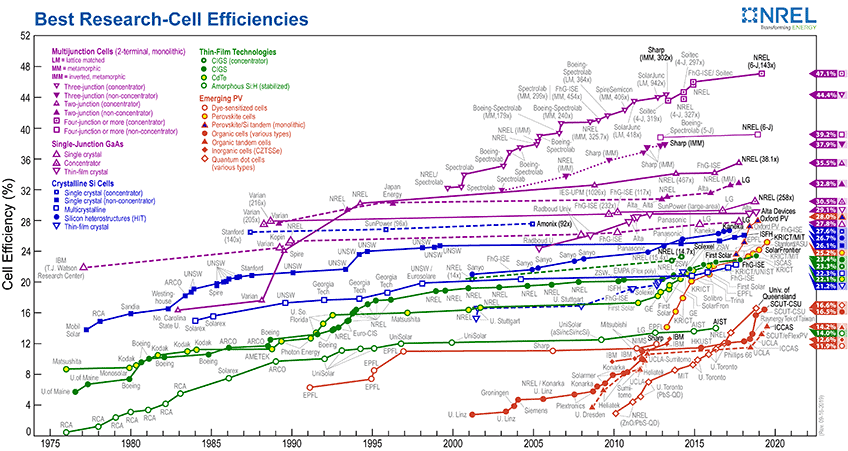
For more information on their screening procedures for researchers who make it onto the graph, visit their website.
We’ve still got a long way to go, though. Solar cells don’t generate emissions during their operation, but we still need to burn fossil fuels to make them. All energy sources have these so-called indirect emission equivalents, measured in grams of CO2 per kilowatt-hour. To compare all the different methods of energy production fairly, a case study of power plant generation calculated a lifecycle greenhouse gas emission of 53 g CO2-eq/kWh for a photovoltaic power plant, compared to 975 g for a coal-fired plant, 742 g for an oil-fired plant, 24 g for nuclear, 11 g for hydro, 15 g for geothermal, and 29 g for wind.
It is also worth considering that solar cells are often made using some materials which are hard to handle and recycle.
In order to develop solar cells to their maximum potential and mitigate their disadvantages, we need to understand in detail everything that’s happening between materials, electricity, and light.
In our next chapter, we will examine the theory and fundamentals of a solar cell.